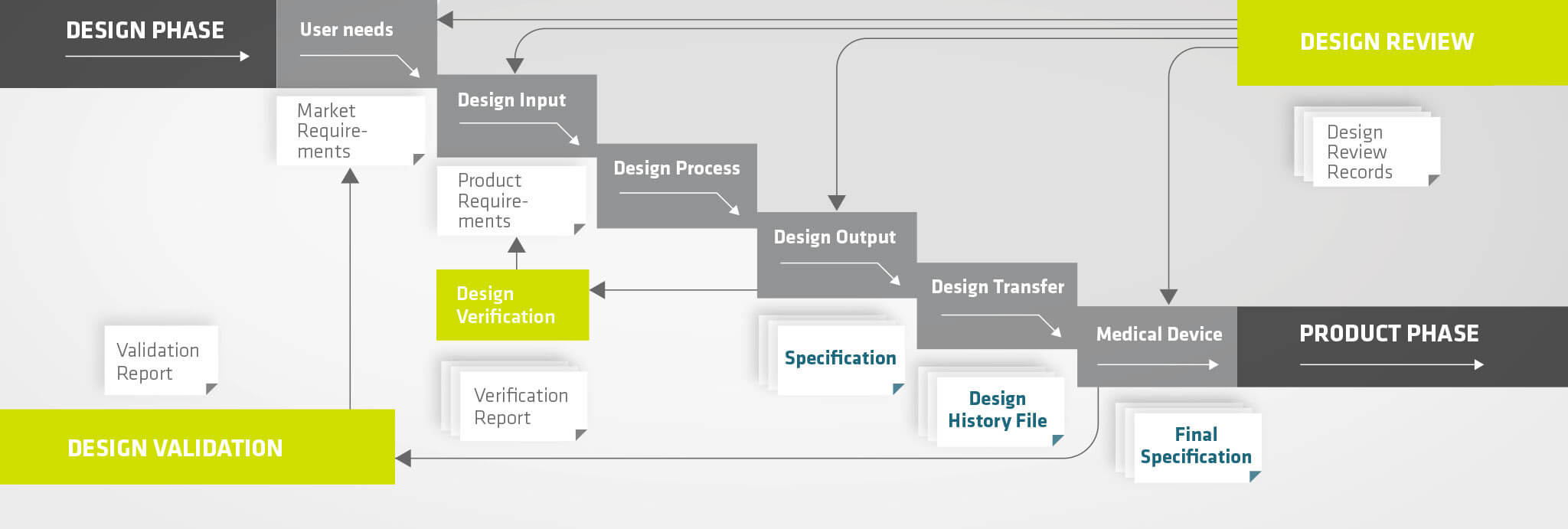

Our development process is based on the following pillars:
-
Design Control Process (FDA 21 CFR 820 and ISO 9001)
-
DIN EN ISO 13485
-
VDI guideline 2221
-
Simultaneous | Concurrent Engineering
-
Our invenio experts' many years of experience
On this basis, we offer you development in full with the best possible flexibility. We adapt to your specific requirements and use them to create templates, gates and reviews. In addition, we provide work patterns that can be protected – the rights are of course passed on to our customers.
Through the close cooperation between the interdisciplinary development teams with the Laboratory and Quality Services teams, we deliver fast and effective solutions on a broad basis.

The transfer of our know-how to you is the core of a successful development for us! To ensure this, you are always pro-actively informed about the project status and details of development. We also support you in interdisciplinary communication, including quality management, risk management and production planning. For important decisions, we prepare a decision template and actively involve you in the process.
Our invenio development process is conceptually divided into five phases:
- Requirement Phase (User Needs)
- Specification Phase (Design Input)
- Design Process
- Design Verification and Transfer
- Design Validation
Already in the early discovery of innovative products or expansion of your portfolio, we support you in the generation of ideas, the preliminary studies and the determination of requirements.
-
Preliminary study and pattern creation
-
Requirements Engineering (user and product requirements, regulatory requirements, standards and laws)
-
Risk Analysis and Management
-
Creating detailed specifications
The 'Specification Phase' includes, among other things, the creation of concepts using situationally selected methods and the testing of viability. The goal is to record the later technical implementation in the specification.
-
Innovation Workshop
-
Methodical Concept Development
-
Analytical Testing of Viabilityt
-
Early Preliminary Tests to evaluate and select the concept approaches
-
Consideration of conformity to product-specific and harmonized standards
-
Derive the Validation and Verification plan (VVP) for the organization of requirements and their review
-
Review the development in terms of Risk Management and Implementation of accompanying FMEAs by the Quality Services Team
After reviewing the requirements specification, we will translate your requirements into a suitable design – including the step-by-step verification of the design specifications. invenio offers everything from one source: Mechanics, electronics and software.
-
Design, analytical and numerical calculation (FEM) and simulation
-
Create electronic block diagrams, isolation diagrams, schematics and layouts
-
Component search and production of the prototype board including programming of the software
-
Set up handling or usability patterns
-
Imaging of individual and overall functions in the context of functional and laboratory samples
-
Development-accompanying testing for risk minimization and pre-verification according to the product-specific Validation and Verification plan (VVP)
-
Mechanical and electrical tests to verify compliance with standards (eg IEC 60601-1, IEC 61010-1) or specific requirements
-
Early involvement of Production Planning, Risk Management, Quality Management and other units throughout the Design Process
In 'Design Verification', the comparison between design input and output on the final prototype is carried out by testing the quantifiable product requirements from the specification. With the successful completion of the tests, the entire development results are transferred to you in a final structured handover and the start of production is supported.
-
Production, sampling and assembly of a first pilot series for testing
-
Verification of design input and design output by qualified tests and experienced testing experts
-
Testing according to harmonized and product-specific standards
-
Documentation in invenio or customer-specific test reports
-
Transfer of the specifications to your or our production
-
Development of devices for production, assembly and testing equipment
-
Creation of assembly instructions and test instructions
invenio also serves as a competent partner in the field of validation.
-
Creation of test cases, questionnaires and evaluation of the field test
-
Technical support of field devices and products
-
Derive requirements and solutions from user feedback
Would You like to contact us?
Gladly we are at your disposal for any questions!
