invenio develops active and non-active medical devices of all risk classes according to your individual requirements – from walking aids to implants. Our expertise ranges from mechanical and mechatronic products, electronics and embedded software to stand-alone software. We follow the processes of our quality management system in accordance with ISO 13485 and ISO 9001 and in line with 'Good Manufacturing Practices (GMP, GxP)'.
We offer you the development of your medical devices throughout the entire development process with the greatest possible flexibility. We adapt to your specific requirements and processes and create designs, documents and prototypes. Thanks to the close co-operation – also across the invenio network – between the interdisciplinary development teams with engineers, clinicians and many more as well as our own laboratory and quality services divisions, we deliver effective solutions quickly.
The experience and findings of the last 15 years in medical technology have significantly shaped our own invenio development process and are our guideline in the development of medical products. Today – depending on the task – up to 50 employees in 4 companies can work on your project, whereby you have only one contact person at invenio.
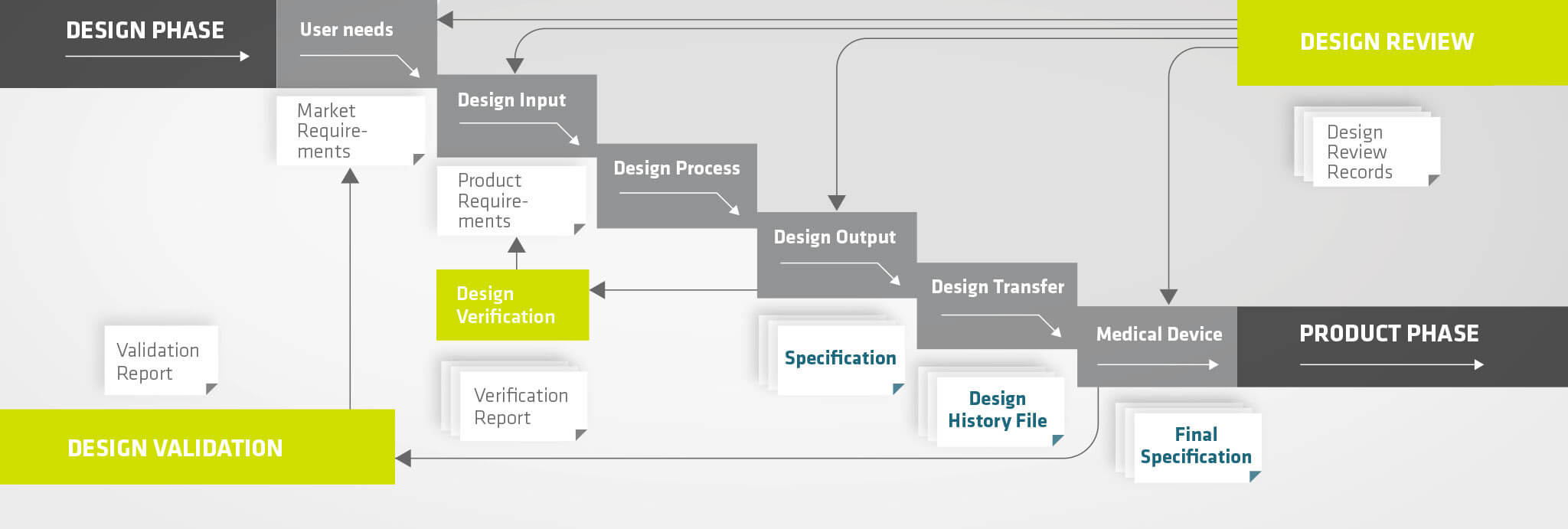
Our approach to developing a medical device – regardless of whether we use our own development process or the customer's – consists of the following steps:
- Requirement Phase (User Needs)
- Specification Phase (Design Input)
- Design Process
- Design Output & Verification
- Design Validation
- Design Transfer
From the early stages of finding innovative products or expanding your portfolio, we support you in generating ideas, conducting preliminary studies and determining requirements.
-
Preliminary study and prototyping
-
Requirements engineering (user and product requirements, regulatory requirements, standards and laws)
-
Risk analysis and management
-
Creation of a detailed specification sheet
The 'Specification Phase' includes, among other things, the creation of concepts using situationally selected methods and feasibility testing. The aim is to record the subsequent technical implementation in the specifications.
- Innovation workshop
- Methodical concept development
- Analytical feasibility testing
- Early-stage usability to evaluate the clinical process
- Early preliminary tests to evaluate and select concept approaches
- Consideration of conformity with product-specific and harmonised standards
- Derivation of the validation and verification plan (VVP) to organise the requirements and their review
- Reviewing the development with regard to risk management and carrying out accompanying FMEAs by the Quality Services team
After reviewing the specifications, we translate your requirements into a suitable design - including the step-by-step verification of the design specifications. Invenio offers everything from a single source: mechanics, electronics and software.
- Design, analytical and numerical calculation (FEM) and simulation
- Creating electronic block diagrams, insulation diagrams, circuit diagrams and layouts
- Component search and production of the prototype board including programming of the software
- Creation of handling and usability patterns
- Mapping of individual and overall functions as part of functional and laboratory samples
- Testing during development to minimise risks and pre-verification in accordance with the product-specific validation and verification plan (VVP)
- Mechanical and electrical tests to check conformity with standards (e.g. IEC 60601-1, IEC 61010-1) or specific requirements
- Early involvement of the production planning, risk management, quality management departments and other units during the entire design process
Design verification involves comparing the design input and output on the final prototype by checking the quantifiable product requirements from the specifications. Once the tests have been successfully completed, the entire development results are transferred to you in a final structured handover and the start of production is supported.
- Production, sampling and assembly of a first pre-series for testing
- Verification of the design input and design output by qualified tests and experienced test experts
- Testing in accordance with harmonised and product-specific standards
- Documentation in invenio or customer-specific test protocols
- Handing over the specifications to your or our production department
- Developing the devices for production, assembly and test equipment
- Creating assembly instructions and test instructions
invenio is also a competent partner in the field of validation. We examine both the fulfilment of the medical purpose and the usability of the medical device.es.
- Creating test cases, questionnaires and analysing the field test
- Deriving requirements and solution approaches from user feedback
invenio supports you in the successful transfer of your medical device from development to series production. We ensure that all design specifications and regulatory requirements are met.
- Creation and review of all necessary production documents and validation of production processes to ensure consistent product quality
- Close cooperation between development, production and quality assurance to identify and implement optimisation potential.
Discover Our Comprehensive Services in Medical Technology Development
Talk to our Experts in the Field of Medical Technology Development
Would you like to contact us?
Gladly we are at your disposal for any questions!




